Life sciences companies face enormous challenges to comply to the increasingly complex regulatory environments. More and more companies adopt the fully electronic eCTD submission format. In the meantime, the industry is already preparing for major revisions to the way in which information is provided to the health authorities. So, what is next and how will it impact both the sponsors and health authorities alike?
An introduction to the Regulatory Product Submission Standard
The short answer is the Regulatory Product Submission or RPS which is a Health Level Seven (HL7) standard, and has been in development for many years already; since June 22nd 2005 to be precise. The good news is that the new standard is being developed with input from all sides that will be affected: the global health authorities themselves providing input into how the information should be best provided to them; the sponsors to provide feedback on how those changes would impact their businesses; and the technology solution providers to ensure that whatever is agreed is feasible from a solution perspective. This collaborative approach to the design should make for a less painful adoption of the standard compared to the original eCTD for example.
The life sciences industry has taken on board as many lessons learned from the implementation of the current eCTD standard, and is delivering a new ideology for the sharing of information between sponsor and health authority. This new approach will improve many of the shortfalls in communication and ultimately help ensure that new products are brought to market as quickly and efficiently as possible in the future, to the benefit of all parties including, at the end of the day, the general public.
What is RPS?
The Regulated Product Submission is a standard designed to simplify the processing and review of regulated product information.
The RPS standard does not touch on the content (which will continue to develop with each new iteration of the eCTD) that is provided but the way in which the dossier will be submitted and how the communication will take place between sponsor and health authority during and after the review.
What challenges does RPS address?
As elements of RPS are incorporated into newer versions of the eCTD, the biggest headaches should be for the health authorities and solution providers who will need to adapt their solutions and processes to meet the new standards. There will inevitably be an impact to the sponsors as well, but most current eCTD solution providers are well underway in adapting their technologies to the RPS elements that will begin to be included from eCTD version 4.0.
RPS has also been designed with the aim of having one common standard for many types of products; not just Pharmaceutical products, but also food additives, medical devices and radiologics, human therapeutics, biologics, tobacco products and veterinary products. The standard’s concepts could make it into the submissions for those products types as well in the future.
One of the other major aims of RPS is to allow for communication between sponsor and health authority. The correspondence that currently requires systems and processes of their own (for both sponsor and authority) will become a part of the same submission lifecycle process; requests for additional information, meeting minutes, and approval communications all being sent as RPS standard messages back to the sponsor by the health authority for example. All information relating to the lifecycle of the product in one place, instead of spread across multiple disparate systems.
Some of the other challenges that RPS addresses include:
- Removing the need to resubmit the same content multiple times by providing the ability to cross reference content that the sponsor has already submitted in a previous filing (there were workarounds for this deficiency in some regions, but RPS has been developed with this in mind from the outset)
- Aiding communication further by including the ability to categorize the content being submitted.
- Improving global roll-outs of products by supporting multi health authority submissions.
So, what is eCTD 4.0?
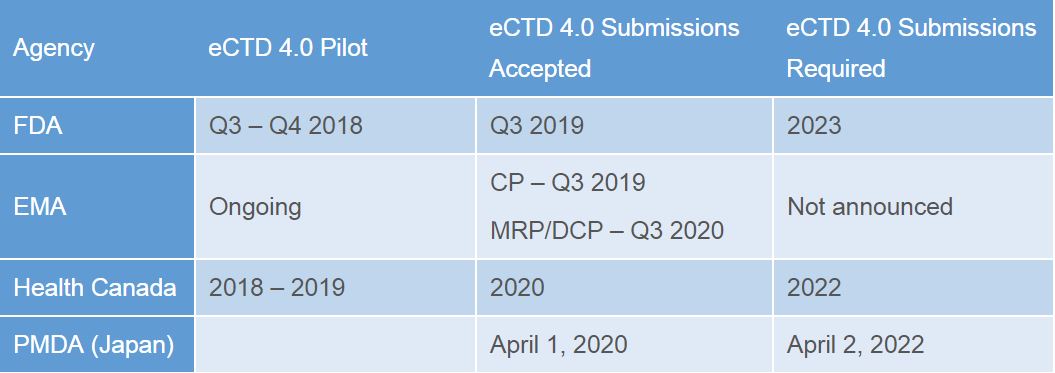
eCTD 4.0 is the first version of eCTD that implements the standards defined in RPS. Different regions are implementing this in their own timelines, and with a different scope (not all regions are implementing the two-way communication between sponsor and health authority for example). The complex initiative of IDMP and its impact on regulatory as a whole has undoubtedly affected the implementation timelines for eCTD 4.0, but the table below outlines some of the regions current plans.
All dates are naturally subject to change as these and other health authorities finalize their implementation guides (the above all have drafts).
How will eCTD 4.0 affect you?
The publishing solution providers will be updating their solutions to handle the eCTD 4.0 specification and the aspects of the RPS standard that are incorporated within. Sponsors need to ensure they understand what is required of them and ensure that their organisation has the necessary technology and processes in place. Taking advantage of the various pilots being planned is something that more sponsors are leveraging than ever before. There are many organisations that provide readiness services to ensure an organisation is well prepared for the changes ahead, and as the various pilot programmes approach many sponsors have begun to leverage these resources to help with their own plans.
This is also a time for sponsors and health authorities alike to review their respective current solutions: the best publishing solution for a sponsor’s specific eCTD 3.2 needs might not necessarily still be the best choice for eCTD 4.0 and the new RPS features that it includes; and likewise, the best tool to review published and submitted content might no longer be the most ideal for the agencies as the way in which communication between them and sponsor changes. Most will undoubtedly look to their existing providers, but as is already being noticed, a large number are beginning to look for alternatives in much the same way as when eCTD was first introduced.
For more information on eCTD v4.0, please check out our latest blogpost: What is eCTD 4.0, and what are the key changes?
Want to know more about eCTD or RPS?
This is the first in a series of blog articles aimed at providing a simplified view of some of the current initiatives taking place in the industry. There are many articles online that provide more detail about eCTD 4.0 and RPS and we will cover updates of some of that detail in the future too, along with articles on the benefits to all affected parties.
For now, you can read the following informative articles:
- A closer look into the eCTD triangle and its modules
- Frequently asked questions about the eCTD format (part 1)
- Frequently asked questions about the eCTD format (part 2)
- eCTD 4.0: Key Changes & Impact on Submission Content Preparation (Article from July 2022)
Do you have content that needs to be transformed into fully compliant, submission ready formats?
DocShifter has your future compliant & submission ready PDF solution.
Did you enjoy this blog post? We have more free knowledge-articles available for you.
Thank you very much for reading our blog post, we hope you enjoyed it.
For more free articles on regulatory topics (eCTD, regulatory submissions, RIM, eCTD v4.0, etc.) we invite you to join our free LinkedIn Regulatory Community.
This community is where we share tips & tricks, updates and learnings on regulatory topics. The community currently has 2500 members and is growing quickly.
-
The PDF format specifications checklist for FDA submissions.
- eCTD 4.0: Key Changes & Impact on Submission Content Preparation
If you think this is interesting, I would like to personally invite you to join the Regulatory Professionals Community on LinkedIn. And don’t worry, it is completely free.
About DocShifter
Speed, quality, scalability, and configurability are reasons why Life Sciences organizations choose DocShifter to generate technically compliant, submission-ready PDF. High volume, high-quality document conversion, on-premises, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce risk. Reduce IT infrastructure costs.
Bonus: We created an FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.