The Life Sciences industry is dedicated to protecting the health of millions of individuals worldwide. The industry is also looking to improve their lives with affordable and easily accessible medicinal products. Patient and patient safety always comes first, which is why the process of bringing a drug to the market takes many years (from 8 to 14 years).
To be able to launch a product, Life Sciences companies must comply with many regulatory requirements, starting from the first stage of research and product development through initial product commercialization.
Migration from paper-based submissions to e-dossiers
The process of submitting documents to regulatory authorities to receive approval for launching a new medicine dates back to the 1940s. Initially, these submissions were all on paper and consisted of small single-volume dossiers containing fewer than 100 pages. Over time, paper dossiers increased considerably both in complexity and size, making paper-format submissions no longer an appealing option.
The accessibility to information technology and the fast broadband Internet connection changed the way documentation and data were submitted. First introduced in 2003, the eCTD (electronic Common Technical Document) became the expected format for electronic submissions to Health Authorities (HAs) worldwide.
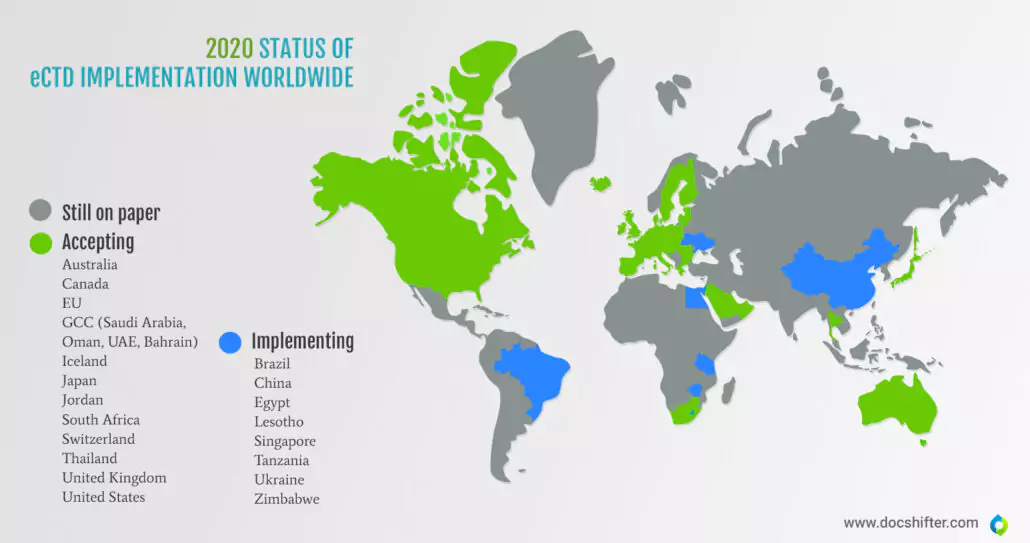
However, the implementation process of the electronic submissions (both eCTD and NeeS – non-eCTD electronic submissions) by the global HAs took many years and was met with reluctance from the industry. In 2020, there are still 80 countries worldwide that file their submissions on paper. Even China, one of the most technologically advanced countries in the world, is only now implementing the eCTD submission format. Currently, the following countries accept eCTD submissions: the US, EU, Canada, Gulf Cooperation Council (Saudi Arabia, Oman, UAE, Bahrain), Jordan, Australia, Switzerland, South Africa, Thailand, and Japan.
PDF vs. Data : The upcoming battle in eCTD submission format
Many other countries like China are just now implementing eCTD. To justify all that investment and effort, it will take them years before looking into alternatives to document and PDF submissions. The many regulations make the Life Sciences industry slow moving and understandably risk-averse. Every new process has to be approved, then validated, and finally implemented and this takes up many years.
However, some general discussions about the ongoing implementation process of IDMP (the European regulations on Identification of Medicinal Products) revealed a possible future alternative to the unstructured PDF format. IDMP is an initiative for patient safety and heavily focuses on structured data. Even though it has nothing to do with launching a new product, many companies hope that IDMP will be the beginning of a new era and allow electronic submissions to evolve into data submissions.
While there is excellent potential to embrace data submissions, the Life Sciences industry is not ready for such a radical change in today’s reality. Just consider that not long ago, companies used to deliver hard-drives and laptops to the HA where the electronic submissions were stored. This process has evolved gradually over a long period of time, and nowadays, any type of electronic submissions is mostly filed in a PDF format. Given the slow adoption speed and the unrealized potential of eCTD & documents, we believe that PDF will be there for many years to come.
Today’s reality in eCTD submission: PDF
There are many reasons why PDF documents are critical for electronic submissions. Let’s take a moment to see what the eCTD stands for: electronic Common Technical Document. This means that in today’s reality, the worldwide expected format for digital submissions is a document, and not data. While that may change in the future, the creation of a new and improved version for the eCTD, namely eCTD 4.0 only comes to support the idea that documents are not going away and that PDF is here to stay.
Another reason why PDF documents are still crucial for electronic submissions is closely related to the huge volumes of documents that are piled up, especially in the first stages of research, development and testing of a new medicinal product. Imagine how many documents a company has after 14 years of research.
When filing for marketing authorization of a new drug, the company must ensure that each and every one of those documents that will be submitted, is technically compliant with the HA requirements. While that is not an impossible task, it is extremely time-consuming to do it manually and let’s not overlook the increased risk of human error. This is where a solution like DocShifter comes in: automatically converting all those documents into submission ready PDFs for any health authority in the world.
How are the current eCTD submission formats expected to evolve in the future?
Let’s consider the countries that are currently accepting eCTD submissions. As we mentioned before, the Life Sciences industry, due to the risks involved, is very reluctant to change. Therefore, it took decades to move to the electronic PDF format. Additionally, making this change into electronic submissions took a lot of effort and time from both HAs and companies.
- On one hand, there are companies that must be able to create the documents in the required eCTD format while being compliant with any additional requirements imposed by each national HA.
- On the other hand, there are the HAs that had to implement the technology needed to be able to review the electronic submissions and also train their employees on using the new technology.
Just consider the financial investments of companies and HAs currently accepting the eCTD submissions or are in the early implementation stages. They would have to cover all costs if they were to move to a new, uncertain and not tested format like data submissions.
It is always good to think about new possibilities, and data submissions can become a reality in the future. However, the era of PDF submissions is still here and is not going anywhere for many years to come. There is still a lot of room for improvement in existing submission processes, starting from how compliant PDFs are generated for submissions.
This is why the Life Sciences industry should focus on the present and search for better ways to optimize their PDF creation processes for electronic submissions. The creation of fully compliant, submission ready PDFs can be much easier, quicker and with less-risk. Multiple compliant PDFs can be generated simultaneously from 1 source document, simplifying the process of submitting the dossier to multiple HAs.
Even if parts of what is submitted in PDF will become data gradually, it is still years away from becoming the norm.
Did you enjoy this blog post? We have more free knowledge-articles available for you.
Thank you very much for reading our blog post, we hope you enjoyed it.
For more free articles on regulatory topics (eCTD, regulatory submissions, RIM, eCTD v4.0, etc.) we invite you to join our free LinkedIn Regulatory Community.
This community is where we share tips & tricks, updates and learnings on regulatory topics. The community currently has 2500 members and is growing quickly.
-
The PDF format specifications checklist for FDA submissions.
- eCTD 4.0: Key Changes & Impact on Submission Content Preparation
If you think this is interesting, I would like to personally invite you to join the group. And don’t worry, it is completely free.
About DocShifter
Speed, quality, scalability, and configurability are reasons why Life Sciences organizations choose DocShifter to generate technically compliant, submission-ready PDF. High volume, high-quality document conversion, on-premises, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce Risk. Reduce IT infrastructure costs. Rated 5 starts on Gartner’s Capterra platform.
Bonus: We created a FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.
Last update: 08/01/2021