1. Do not wait until the last minute to resolve content-related issues
A typical drug development process takes from 5 and 15 years, and documentation is created every step of the way. Marketing Authorisation submissions are compiled at the end of this timeframe. Documents are typically only checked for compliance either just prior to or after a draft submission is published. What if your original source document needs to be updated?
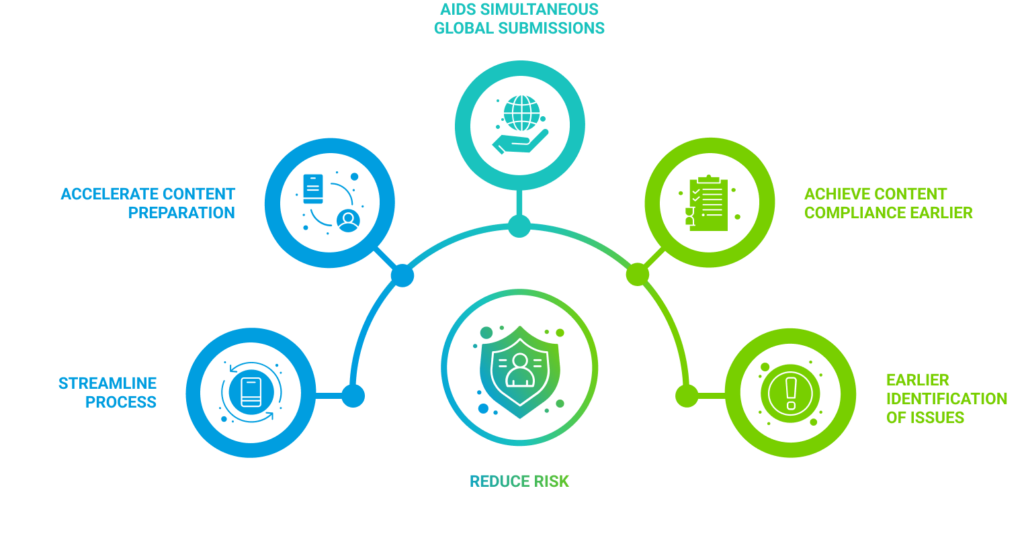
What we mean by achieving compliance earlier, is to ensure that any issues are identified much earlier in each document’s lifecycle: at the time the source file is saved; or at the time the PDF version is created. It means using a solution to generate fully technically compliant PDFs as soon as the source document is available, and not relying on other tools and plugins to achieve that level of compliance. Any remaining issues that require an update on the original source document can be identified automatically at source or as the PDF is generated. Rather than manually checking each PDF is submission ready, that check-list can be automatically created during the process.
The message we try to get across (and where our customers see the biggest difference) is that by achieving document compliance earlier in this way, you can reduce the risk of missing submission deadlines and of issues from having manual steps in your process. Problems can be addressed weeks, months, or even years earlier in the development process and the entire submission creation process simplified.
2. Reduce the number of steps needed in your submission content preparation
When examining the submission content preparation process and its implications, it becomes clear how complex and time-consuming it can be.
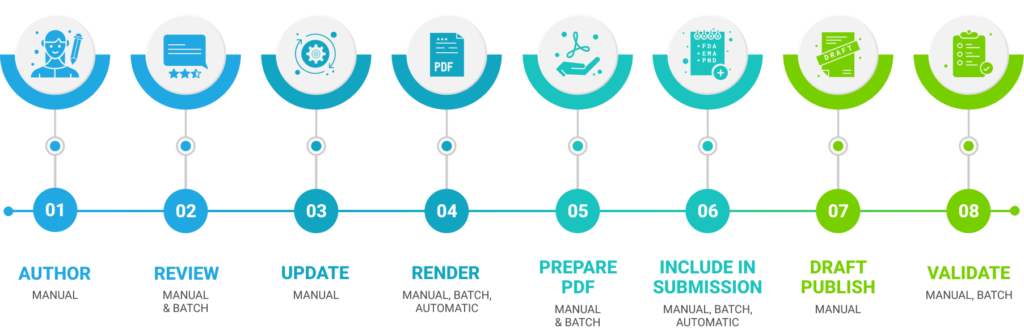
Let’s consider the scenario where 3 source documents are included in Dossiers, and each document must be rendered to a PDF specification. These PDFs then need to be prepared for submission in various countries, including the US, Europe, Japan, Switzerland, and Canada. This alone adds 15 additional steps to the process. Moreover, each PDF must be meticulously checked for submission readiness, as the initial rendition requires further manipulation. Any issues discovered during this stage must be addressed, and the PDFs need to be incorporated into the respective submissions.
Following that, draft versions of each region’s submission are created, and extensive validation is performed to identify content-related and submission-related issues. It is essential to iterate and potentially republish until a final version is achieved. Even without major changes or issue resolutions, this process already involves 86 steps for three documents across five countries and health authorities. With just one resolution per document, the count rises to 116 steps, considering the possibility of multiple actions within each step.
Therefore, the number of actions required can significantly exceed 116. As the content is handed off to regulatory operations, the focus shifts to ensuring timely delivery, compliance, and readiness for inclusion in the submission. Deadlines and compliance concerns loom large throughout, making it imperative to address potential issues in the source document. Unfortunately, this remains a highly manual undertaking.
3. Knowing where to look for in your PDF content gives you a competitive advantage
It was one of our customers that opened our eyes. We spend a lot of energy talking about how good we are at ‘rendering’ or ‘converting documents to PDF’. Until a customer told us before the webinar that PDF conversion is the easy part. And not the problem.
“The problem is not actually rendering. We create those PDFs anyhow. The problem is us spending hours and hours of time on editing PDFs with tools like ISIToolbox. Especially not knowing what was wrong with the PDFs. And that is what DocShifter is now doing for us.”
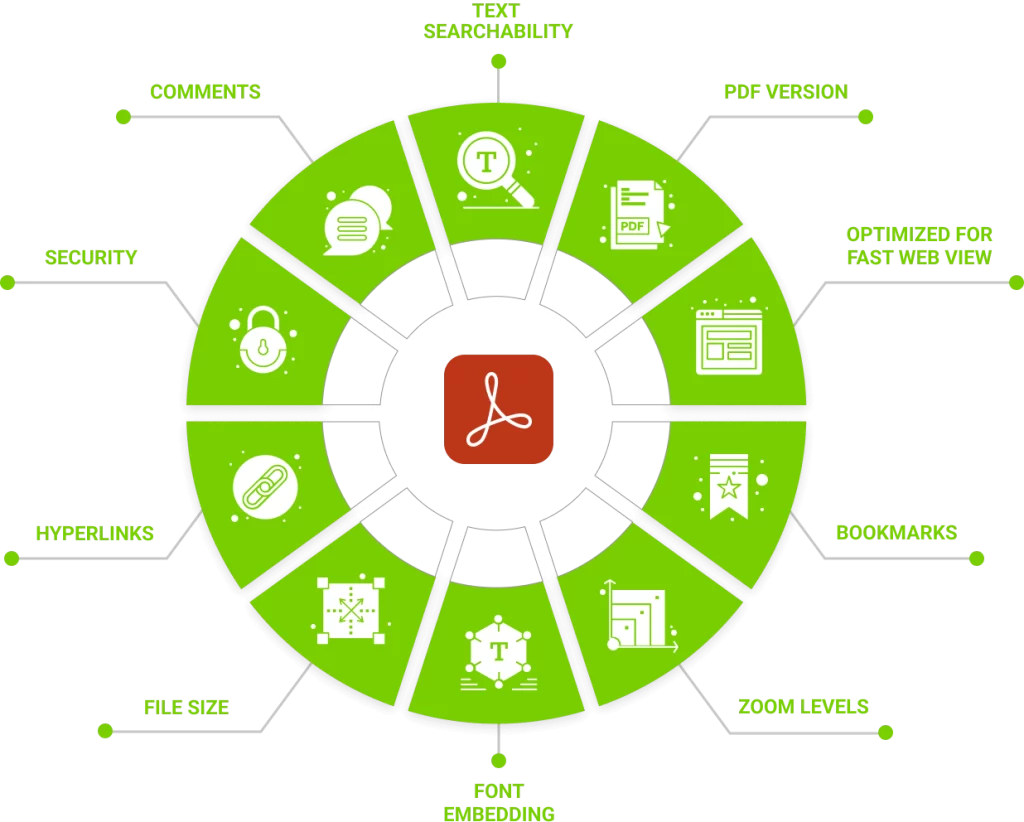
DocShifter goes beyond PDF rendering and enables the identification and resolution of issues in Microsoft Word and PDF documents. DocShifter can be configured to perform checks for comments, paragraph-related problems, section numbering discrepancies, bookmark and table issues, font-related issues, hyperlink problems, and image-related concerns. The generated reports provide detailed information on the checks performed, compliance status, resolved and unresolved issues, and the location of each problem: Significantly speeding up your review and correction process, while reducing manual effort and minimizing errors.
The manual nature of the existing processes amplifies the challenges faced by life sciences organizations. Identifying issues in the original source document is a time-consuming task, often involving manual scrolling and visual inspection. Common issues include incorrect section numbering, improper hyperlink usage, table formatting problems, and technical issues related to font embedding. Similarly, when validating PDF content, concerns arise regarding bookmarks, hyperlinks, zoom levels, table of contents, PDF properties, font embedding, and initial view settings. These checks are typically performed manually, using batch tools or Acrobat plugins, leaving room for human error and increasing the overall processing time.
4. Benefits of automating compliant PDF rendering
In terms of benefits, one of the key advantages is the ability to streamline the content preparation process for different health authority submissions. By utilizing capabilities that allow you to prepare the same content multiple times, you can significantly accelerate the overall process. This ultimately contributes to the goal of facilitating simultaneous global submissions and achieving content compliance much earlier in the process. With DocShifter, you can validate the content and address any issues a lot sooner, eliminating the need to wait until the last moment to identify certain types of problems. This early detection and resolution save valuable time. The primary aim is to minimize the risk of missing deadlines and ensure that submissions are free from non-compliant elements. In essence, this approach offers several benefits by enabling content compliance to be achieved earlier in the workflow, rather than realizing it only at the final stages after draft publication.

How to automate PDF rendering for global submissions with DocShifter (with a practical demo)


![[Webinar Summary] How to automate PDF rendering with DocShifter featured image [Webinar Summary] How to automate PDF rendering with DocShifter featured image](https://test.docshifter.com/wp-content/uploads/2023/06/featured-image-1-6489aedde2b47.webp)