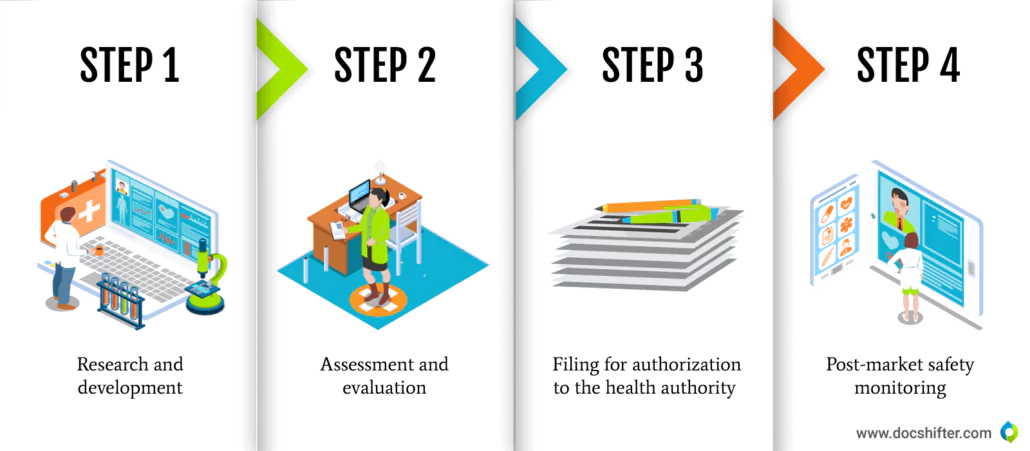
Bringing a new medicine or a product to the market is a complex process that typically takes from 8 to 14 years. Although this process may vary from one Health Authority (HA) to another, there are some general stages that the medicine must go through before it can be safely distributed to patients. It starts with research and development (R&D), then slowly moves into the next steps, including assessment and evaluation through non-clinical and clinical trials, filing for marketing authorization to the HA and getting regulatory approval. The final stage is the post-market safety monitoring after the product is launched to the market.
Patient safety is crucial in the Life Sciences industry
There are high risks of taking any shortcuts in this long process. In fact, a study conducted by the Tulane University back in 2003 showed that each 10-month reduction in the US Food and Drugs Administration (FDA) review time period for a new drug can lead to an 18% increase in serious adverse reactions, an 11% increase in drug-related hospitalizations, and a 7.2% increase in drug-related deaths.
Patient safety is the reason why the process of bringing a new drug to the market takes so much time. And since it is the main focus of the entire Life Sciences industry, the new drug can be rejected even before filing for marketing authorization to the HAs if it isn’t considered safe for public use. For example, during the clinical trials, the first phase sees approximately 70% of drugs move to the next step. Phase III trials see only 25-30% of those remaining drugs enter the final stage leading to HA review.
Filing for marketing authorization – is success guaranteed?
Once the drug passes the testing phase, pharmaceutical companies finally reach the stage of submitting a request for authorization to the HA where the drug is going to be marketed. However, there is always a risk of getting a rejection from the HAs.
What is refusal to file (RTF)?
Refusal to file is an official letter issued by the health authority to inform a sponsor that their application doesn’t comply with the compliance needs of the agency. In other words, refusal to file is the agencies way of saying that they will not be reviewing the sponsor’s submission application.
Research shows that 25% of the marketing applications for drug authorization submitted in the past to the European Medicines Agency (EMA) were rejected. Additionally, looking at the statistics published by the FDA, a total number of 73 refusal-to-file (RTF) letters were issued to non-compliant pharmaceutical companies over a period of 7 years.
However, some companies were able to turn it around and had their applications reviewed after receiving an RTF initially. Some even got approvals, after meeting FDA’s demands.
How can I avoid a refusal to file from FDA?
One of the main reasons of Refusal to file is the inability to comply with the technical HA requirements.
As explained in one of our previous articles, it takes many years for a new drug to be approved. When the pharmaceutical company is finally able to file for marketing authorization, it must ensure that each document from the millions collected over so many years of research respects all the specific technical requirements from each national HA. This is where an automated document conversion solution like DocShifter can help companies: automatically convert all those documents into submission ready PDFs for any HA in the world and reduce the risk of receiving a Refusal to file.
What are the consequences of receiving a Refusal to file from the FDA?
When a Life Sciences organization’s marketing authorization for a new drug is rejected by the HA for non-compliance, the negative impact can be translated into significant financial losses and damages to the company’s image and reputation. To give you a better understanding of the importance of compliance in the Life Sciences industry, let’s focus on the consequences of receiving an RTF from the FDA.
Rejected FDA submissions could cost a life sciences company between $660,000 to $8,000,000 for every day the drug is delayed.
- Fines imposed by the FDA. Under the Prescription Drug User Fee Act (PDUFA), when applying for the approval of a new drug, the pharmaceutical company must pay an application fee (for 2020, the current rates are: $1,471,483 – if no clinical data is required, and $2,942,965 – if clinical data is required). If a drug application is refused for filing, the pharma organization can receive back only 75% of the application fee, incurring financial penalties of $367,871 up to $735,741.
- Loss of R&D financial investments. Statistics in the industry show that pharmaceutical companies spend, on average, 17% of their revenue on developing a new drug. This amount translates into an average of $4 billion, and an RTF letter delays (months) in getting the product to market and causing loss of revenue. Even when the company takes actions to become fully compliant with the FDA’s requirements, it will still lose significant amounts of money every day that it doesn’t bring the new drug to the market.
- Stock price drops. This is another negative impact that contributes to the significant financial losses incurred after receiving an RTF from the FDA. For example, there was the case with a pharmaceutical company that filed for marketing authorization of its new drug with the FDA in December 2015. Two months later, in February 2016, the company announced that it received an RTF letter from the FDA. Soon after this announcement, the stock prices plummeted by 37.3% in a single day.
- Negative press. Ensuring patient safety is vital in the Life Sciences industry. This means that patients also should be confident in the pharmaceutical company’s ability to bring a new drug to the market that may help improve their lives. If the press discovers that the pharma company’s drug application was refused by the FDA, all that negative buzz can end into losing the customers’ confidence. And when that happens, the consequences can be truly catastrophic and it can really be the end of the new drug, long before being launched to the market.
How can DocShifter help pharmaceutical companies achieve technical document compliance?
Pharmaceutical companies can get an RTF for two main reasons: the inability to comply with content requirements (missing information or summaries, appendixes not submitted, etc.) or with technical PDF formatting requirements of health authorities.
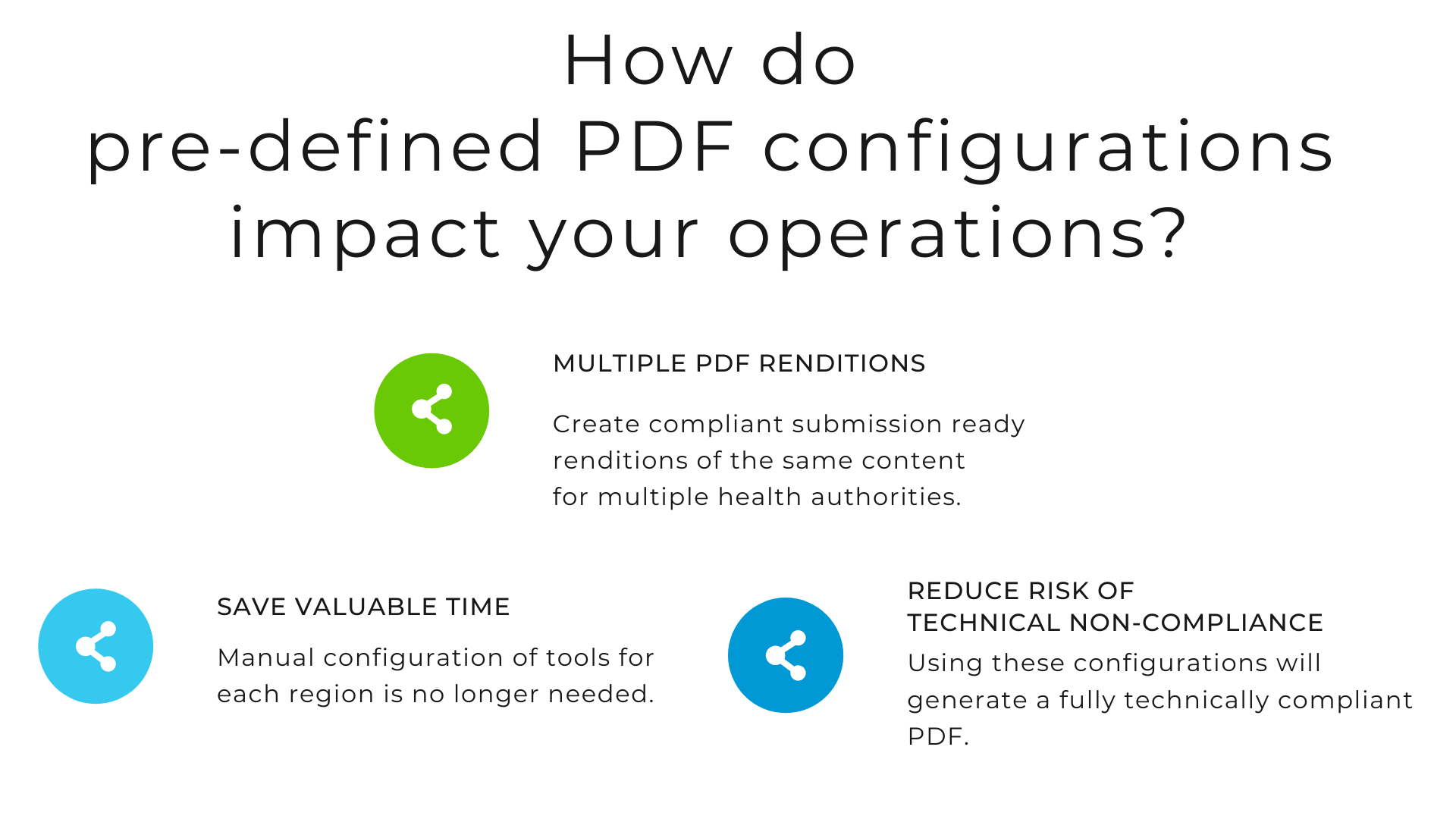
Even though there are some tools that can create compliant submission ready PDF documents in multiple steps, they are costly, time-consuming, and have to be manually configured. The DocShifter document conversion software makes sure that your electronic submission documents are fully technically compliant with any HA. Simple, automated, and fast.
To ease compliance and reduce the risk of Refusal to file, DocShifter software provides technical PDF configurations, that are predefined to the technical requirements of each national HA like the FDA (in the US), EMA (in the EU), PMDA (in Japan), HC (in Canada) and many more. With this, every PDF you generate with DocShifter will exactly match what the HAs demand.
Did you enjoy this blog post? We have more free knowledge-articles available for you.
Thank you very much for reading our blog post, we hope you enjoyed it.
For more free articles on regulatory topics (eCTD, regulatory submissions, RIM, eCTD v4.0, etc.) we invite you to join our free LinkedIn Regulatory Community.
This community is where we share tips & tricks, updates and learnings on regulatory topics . The community currently has 2000 members and is growing quickly.
In the first 2 editions, we focused on:
If you think this is interesting, I would like to personally invite you to join the group. And don’t worry, it is completely free.
About DocShifter
Speed, quality, scalability, and configurability are reasons why biotech, pharma and medical devices companies choose DocShifter to automate and simplify all their document conversion processes. From automatically checking and fixing Word and PDF documents to generating compliant, submission-ready PDF renditions, DocShifter software offers a unique and proven approach to speed up time-to-market.
High volume, high-quality document conversion, on-premise, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce risk. Reduce IT infrastructure costs. Rated 5 stars on Gartner’s Capterra platform.
Bonus: We created an FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.
Last update: 08/05/2021