Introduction:
“Converting to PDF is easy, and everyone does that. The problem is us spending hours and hours of time on editing PDFs with Acrobat, or other desktop tools. Especially not knowing what was wrong with the PDFs. And that is what DocShifter does for us.: fixing all our PDFs automatically, or at least telling us what is wrong and where to look in a detailed report.”
This is the feedback we have recently received from one of our customers. Fantastic! But what did she mean? What did the journey look like for her, and her team? We explore the before and after DocShifter situation in this customer story.
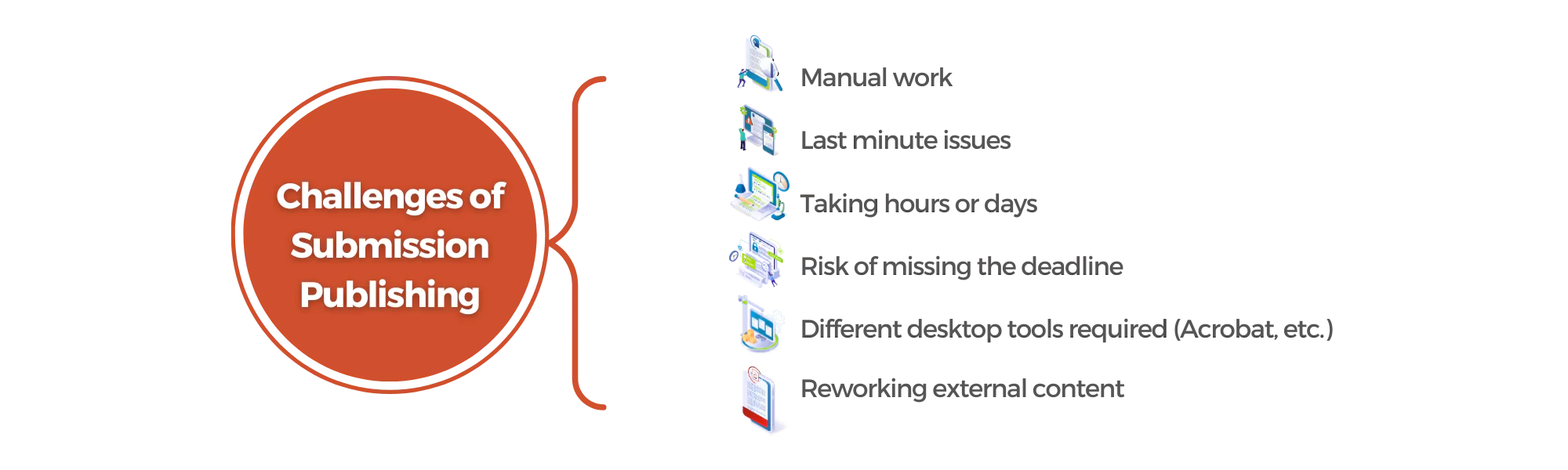
Challenges Faced:
Prior to integrating DocShifter into their Veeva workflow, the company encountered numerous challenges with their PDF documents. Operating within strict regulatory frameworks, regulatory compliance was a top concern. Ensuring that PDF documents complied with specific versions, standardized zoom levels, bookmarks, and searchable content was a time-consuming and error-prone task. Additionally, inconsistencies in PDF formats and versions across multiple stakeholders further complicated the process of compiling regulatory submissions. External content, often sourced from Contract Research Organizations (CROs) and other stakeholders, introduced significant hurdles in standardizing the content and aligning it with internal processes.
These challenges not only impacted the efficiency of their regulatory team but also delayed their time to market.
Manual PDF Checks and Fixes:
Before introducing automation, the regulatory team dedicated extensive hours to meticulously review and correct PDF documents. Flattening PDFs, correcting outdated PDF versions, setting zoom levels and bookmarks, converting images into searchable PDF content, and adding hyperlinks were all painstaking tasks that required meticulous attention to detail. However, despite their best efforts, these manual processes were time-consuming, resource-intensive, and prone to human error.
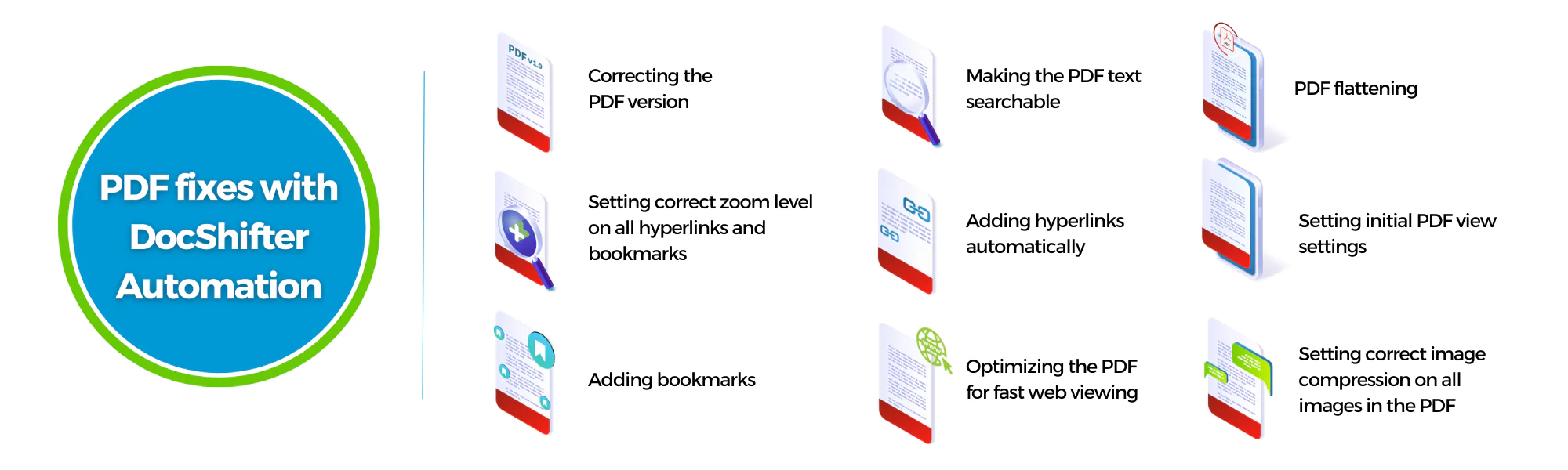
Automatically finding and fixing non-compliant elements in PDF files.
What if there was an automated way of telling the regulatory team where to find the non-compliance elements in their PDF files? And moreover, even automatically fixing those errors in most cases. And returning a clear report on the fixes and the outstanding issues.
This is exactly what DocShifter automation allowed this leading pharmaceutical company to achieve: identifying and fixing many PDF-related errors without any manual intervention.
Here are some of the PDF fixes that DocShifter completely automated.
Benefits of automated PDF check and fixing
60% Time Savings:
The manual efforts previously invested in PDF checks and fixes were significantly reduced. The company estimates a remarkable 60% time savings in document processing, allowing them to allocate resources to more strategic tasks.
30 % Faster Time to Market:
By automating time-consuming tasks, the company experienced an estimated 30% reduction in time to market. The streamlined processes facilitated by DocShifter enabled them to meet regulatory deadlines more effectively and gain a competitive advantage.
Next level Compliance:
DocShifter ensured regulatory compliance by automatically flattening PDFs, correcting outdated versions, and aligning zoom levels and bookmarks according to regulatory specifications. This significantly reduced the risk of non-compliance and improved the accuracy and consistency of their regulatory submissions.
Improved Efficiency and Productivity:
The automation capabilities of DocShifter eliminated the need for manual intervention, allowing the company’s team to focus on more critical aspects of regulatory operations. The streamlined processes enhanced overall productivity and operational efficiency.
Quantifiable Results: 40% less time spent on document processing:
40% reduction in document processing time, translating into substantial cost savings. The accelerated time to market, combined with enhanced compliance and productivity, resulted in a significant return on investment.
Thank you, customer!