DocShifter helps biotechnology company achieve %60 time saving in document preparation and speed up time to market by %30.
All thanks to automated PDF checking and fixing.
Helping solve manual PDF checking forever
Manually checking every PDF in Veeva Vault for submission-readiness
Prior to integrating DocShifter into their Veeva workflow, the company encountered numerous challenges with their PDF documents. Operating within strict regulatory frameworks, regulatory compliance was a top concern. Ensuring that PDF documents complied with specific versions, standardized zoom levels, bookmarks, and searchable content was a time-consuming and error-prone task. Additionally, inconsistencies in PDF formats and versions across multiple stakeholders further complicated the process of compiling regulatory submissions.
External content, often sourced from Contract Research Organizations (CROs) and other stakeholders, introduced significant hurdles in standardizing the content and aligning it with internal processes.
These challenges not only impacted the efficiency of their regulatory team but also delayed their time to market.
The DocShifter impact


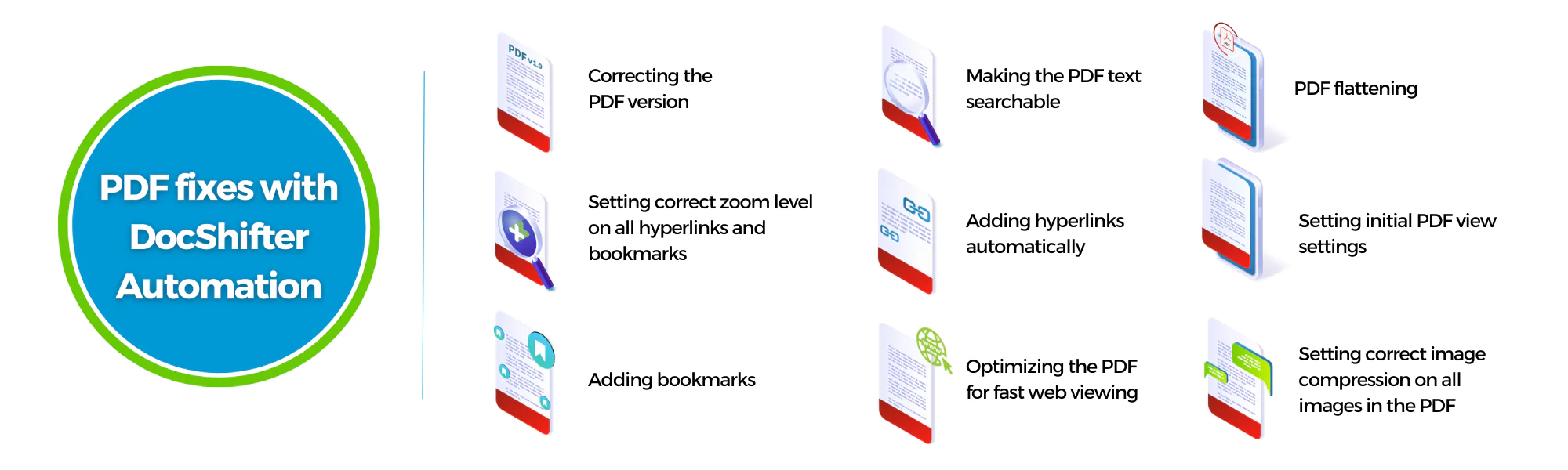
Some of the PDF fixes DocShifter automated
Efficiency gains in regulatory
through automation
Next level Compliance
DocShifter ensured regulatory compliance by automatically flattening PDFs, correcting outdated versions, and aligning zoom levels and bookmarks according to regulatory specifications. This significantly reduced the risk of non-compliance and improved the accuracy and consistency of their regulatory submissions.
Improved Efficiency and Productivity
The automation capabilities of DocShifter eliminated the need for manual intervention, allowing the company's team to focus on more critical aspects of regulatory operations. The streamlined processes enhanced overall productivity and operational efficiency.


