Life Sciences organisations are required to create huge quantities of documentation to support the marketing of their products around the world. Industry standards have been put in place to ensure that Global Health Authorities receive content in a manner that simplifies the review and approval process of new medicines or devices. These guidelines do provide the sponsor organisations with some flexibility, but good authoring processes are essential to ensure the content is presented in both the format and to the technical specifications required by the agencies.
Most health authorities base their specific submission content requirements on guidelines set out by the International Council for Harmonisation (ICH). The final documents being submitted will mostly be in PDF format, but the content will be created and edited using traditional authoring tools such as Microsoft Word. To ensure the PDF is compliant, the Word document needs to be prepared in a specific way. If done correctly, and with the correct tools in place for content conversion, the process of generating the final PDF files for inclusion in regulatory submissions can be much simplified.
The guidelines for content cover many aspects of how the document will look and also how it can most easily be navigated through when being reviewed.
What do some rules regulatory guidance cover? Here are some examples.
- Font Specification
- Recommended fonts
- Permissible font sizes
- When fonts should be embedded
- Use of Images
- Acceptable image compression methods (specific for colour, black & white, greyscale)
- When images should have Optical Character Recognition (OCR) applied
- How the text should flow around images
- Headings
- Section numbering formats
- The sequencing of numbering
- How many heading levels to use
- Linking
- What style should be applied to hyperlinked text
- Allowable target types for links
- General Formatting
- Track changes & comments usage
- Empty pages and other spacing
- Table formatting
- Use of embedded objects
Ensuring these rules are followed on every single document used is very difficult, and often extremely time-consuming for those involved in authoring and preparing the content for use in submissions. It also becomes significantly more difficult to control once content is authored by external contributors. Typically, much of the work to ensure these guidelines are met is still performed manually or using multiple tools to check and rectify specific issues.
How to simplify regulatory content creation issues? 4 Tips to Ease the Burden
1. Correctly implement a good set of document templates.
This sounds logical, but often templates are purchased as a package and not configured in a way to suit the needs of both the company itself and the Health Authorities. Often this means that many formatting and other changes still need to be performed manually once the content has been created.
If purchasing ready-made templates, ensure that the vendor can help configure the templates fully to maximize your investment, otherwise you will not be getting the value you should expect.
While templates can help keep authors on track with the guidance, it is always possible for changes to be made that would mean the document no longer complies with the rules.

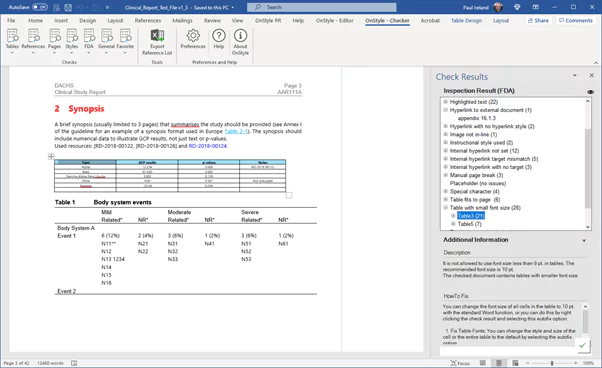
Implementing a number of different tools or macros to help identify issues with the content is very complex and becomes expensive to maintain and manage. If developed in house, the functionality needs to be kept up-to-date, or you have to deal with multiple vendors if sourced externally. Choose a single tool that can more easily be distributed to your authoring community that best meets your specific needs. Tools exist that:
- Can check your content for both internal and external compliance issues,
- Identify where the document deviates from the rules set out in the template,
- Provide the ability to fix the issues individually or in batch,
- Extend the capabilities in Word to ensure compliance is more easily maintained in the first place.
- Are maintained by the vendor to comply with any evolving requirements.
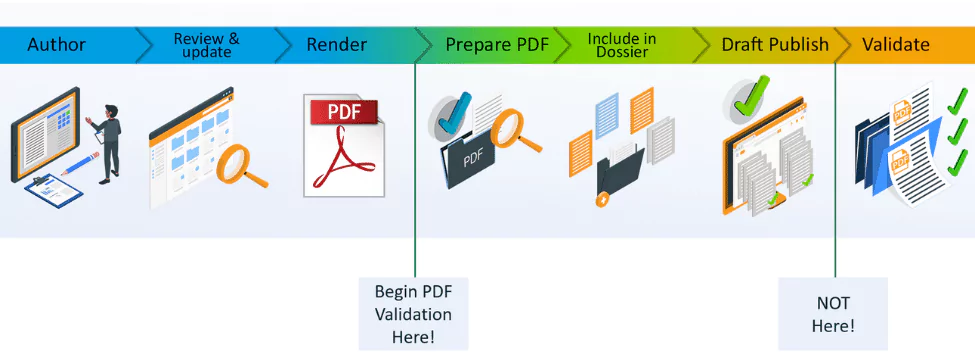
3. Implement a new rendering solution.
Rendering solutions are often put in place alongside the Document Management System and little thought is put into what capabilities they offer other than their ability to convert content to PDF. Modern rendering technology can provide many benefits to the authoring process:
- Ensure the PDFs created are fully compliant with the Health Authority specifications (legacy rendering tools typically create standard PDFs which are not suitable for inclusion in a submission without further manipulation).
- Fonts are embedded correctly.
- Hyperlinks are coloured correctly, even if not correctly set in the original source document.
- The appropriate Headings are converted into PDF bookmarks, even if too many heading levels have been created
- Track changes have a consistent look in the PDF output regardless of differing authoring and Word settings.
These are just examples of how a modern rendering software can automatically adjust elements to account for issues in the original source document. Ideally of course the original document will be authored in a way that minimizes the need for adjustments at the rendering stage, but the rendering platform is a critical part of the content creation process that is often overlooked.
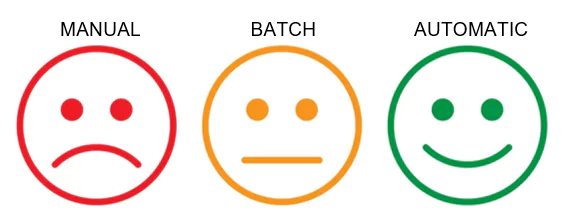
4. Fully automate many of the checks and fixes in the source documents.
The manual and batch methods typically used to perform updates on the documents are highly effective, but do still require an element of human interaction. With any human interaction, a degree of risk is always introduced. It is now also possible to fully automate many of the checks that need to be performed on all documents, as well as fix many of the errors that occur. Without any human interaction.
This automation can be fully integrated into your document lifecycles, whether using a Content Management System or not. Automated workflows will check and fix the documents at the required points in the content’s lifecycle without any involvement from the content creators. This allows your authors and others involved in preparing the content for use in a submission to focus on the content, knowing that many of the formatting and technical issues can be discovered and resolved automatically. It will likely never be possible to fully automate the content preparation process, as there will always be issues that require human interaction. These fully automated tools can at least ensure any issues are automatically identified to be resolved by someone with the correct knowledge later.
Conclusion
Many hours of time is lost by a large number of people to ensure content is prepared with the right formatting and to the right specifications for its intended use. While each check might seem minor on its own, when you multiply the number of checks performed in each document by the number of documents being authored, by the number of versions created through its lifespan, this becomes an enormous issue for any size Life Sciences organisation. Luckily, technology has evolved to ease this burden.
Any automation to these processes can reap huge rewards for any company and ultimately further reduce the risks associated with any manual task. It makes the entire authoring and review process more efficient and ensures the limited time available can be focused- as it should be- on the content and delivering fully compliant content on time.
About DocShifter
Speed, quality, scalability, and configurability are reasons why biotech, pharma and medical devices companies choose DocShifter to automate and simplify all their document conversion processes. From automatically checking and fixing Word and PDF documents to generating compliant, submission-ready PDF renditions, DocShifter software offers a unique and proven approach to speed up time-to-market.
High volume, high-quality document conversion, on-premise, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce risk. Reduce IT infrastructure costs. Rated 5 stars on Gartner’s Capterra platform.
Bonus: We created an FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.