How can you speed up the process of getting your medicinal products approved by health authorities? At the same time, how can you reduce the risk of submitting PDFs that are not complying with technical requirements in your regulatory submissions? Our tips will help you streamline the whole regulatory submission process.
For Life Sciences companies, time is scarce when preparing to launch a new product. Anything that slows down your submission process and introduces a risk of a delay in gaining approval is hugely frustrating. Having your submission rejected by the health authorities because your PDFs don’t meet their technical requirements is time-consuming, financially expensive and negatively affects the company’s image. So here are a few tips on how to tackle this challenge, save time and reduce the risk of non-compliance.
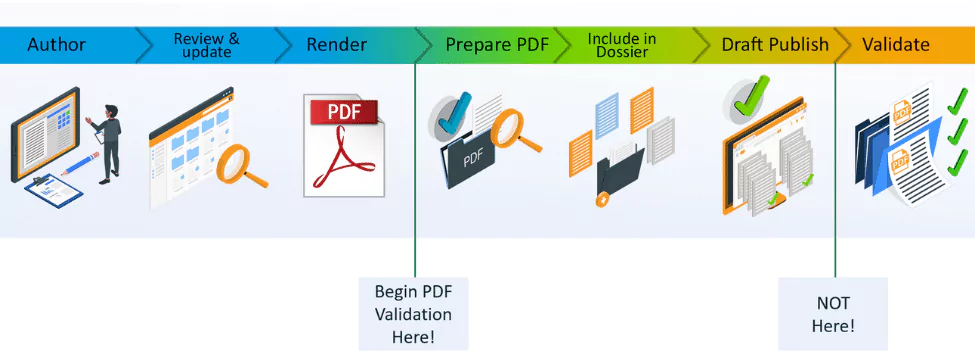
Tip 1: Move compliance earlier in your regulatory submissions (don’t wait until submission time)
When creating PDFs manually or with many rendering tools, you typically need to edit them afterwards in order to meet each of the health authorities’ technical requirements where the content will be used. A regular PDF will not be compliant by default, so additional tools and steps are required to modify each and every PDF created to make it compliant, or wait until the very end of the submission process and let the publishing tool make those adjustments. This approach is time-consuming, means that technical issues are only discovered very late in the process, and increases the risk of making mistakes.
The solution to this problem is to ensure compliance is achieved at an earlier stage in your submission content preparation process. Compliance should actually be reached automatically when you render your content to PDF; not require any additional tools or manual steps, including for multiple Health Authorities if necessary. This enables a shift in focus on quality control, and reduces the number of issues being resolved after the publishing stage.
Tip 2: Automate (reduce the necessity of manually editing PDFs for regulatory submission compliance)
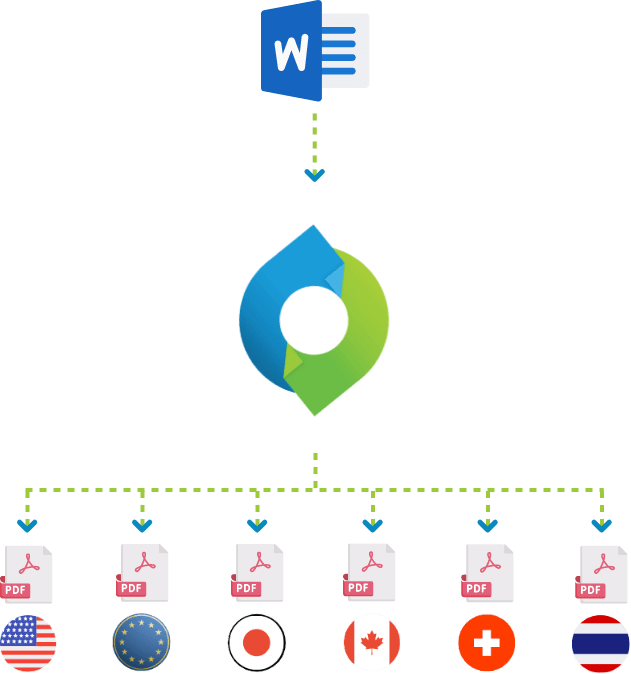
Enhanced efficiency and built-in compliance can only be achieved by using an automated PDF rendering system. Some of these systems can automatically render multiple versions of your PDFs, each meeting the technical requirements of a specific health authority such as the FDA (US), PMDA (Japan), EMA (Europe), Health Canada and Swiss Health. Differences can include the PDF version used, whether the PDFs are optimized for fast web viewing, the number of bookmark levels created and visible on opening, the colour of hyperlinks, whether bookmarks for tables and figures should be grouped together, and if documents exceeding a specific number of pages should automatically have a table of content added.
Many Life Sciences companies are still using desktop tools, such as Adobe Acrobat or DXCToolbox (formerly known as ISIToolbox), to manually edit thousands of PDFs for compliance. This manual editing is extremely time-consuming, repetitive and increases the risk of making mistakes. Automating compliant rendering can help whether you work in a specific market only or if you have a global portfolio of products.
Tip 3: Centralize all your (compliant) PDF conversion/rendering
For a smooth approval process by health authorities, it is necessary to submit PDFs that are consistent technically and visually. This is difficult to achieve when using multiple technologies to create and manipulate your files. Each application can introduce inconsistencies, in particular when they can be configured differently by each user involved.
A powerful system for automated compliant rendering will solve this problem. The PDF rendering engine retrieves the files from all your different controlled content repositories such as OpenText Documentum, Veeva Vault and Microsoft SharePoint, but also from file shares, web services and email repositories, and converts them to consistent and compliant PDFs, ready to be submitted to any health authority worldwide.
DocShifter can help you to reduce the risks of non-compliance, automate and centralize compliant document and PDF conversion.
About DocShifter
Speed, quality, scalability, and configurability are reasons why Life Sciences organizations choose DocShifter to generate technically compliant, submission-ready PDF. High volume, high-quality document conversion, on-premises, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce risk. Reduce IT infrastructure costs. Rated 5 starts on Gartner’s Capterra platform.
Bonus: We created an FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.