Life Sciences organizations deal with external parties and health authorities on a regular basis. Given the amount of information, tracking all that communication is very challenging and burdensome.
“An essential part of any complete RIM platform
is the ability to plan and track all day to day
regulatory activities that an organization needs to undertake.”
These activities are often initiated by a request from a health authority for more information, It is critical that these requests are traceable alongside the tracked regulatory activities in the RIM platform.
Can you keep track of all those incoming and outgoing correspondence?
Information and requests are shared in various formats, by various means and from various locations. Manually keeping track of all this correspondence is a huge burden on time and resources. Organizations still rely on manual processes. Manual processes lead to miscommunication and therefore delays in activities and even fines for missed deadlines.
Central repositories and systems (enterprise content management or document management) to track correspondence are essential elements of the information flow within and outside of life sciences organizations. When information is received from a health authority or external partner (such as a CRO or a medical writing team) it needs to be:
– Processed to uncover what type of correspondence it is.
– Converted into a consumable format based (PDF, Word etc.) on the type of correspondence and who the consumers/responsible parties will be.
– Stored in the appropriate content repositories for the type of correspondence it is.
– Distributed to the appropriate individuals.
Once this has been accomplished, an entry needs to be made in a correspondence tracking system. The company-critical content can then easily be found in the future alongside other information relating to the new regulatory activity. Correspondence is often a catalyst for some form of regulatory activity (such as creating a new submission to provide more information to a health authority for a particular drug).
“Being able to trace the activity from the initial request
provides a fuller picture of the entire
regulatory history of a product.”
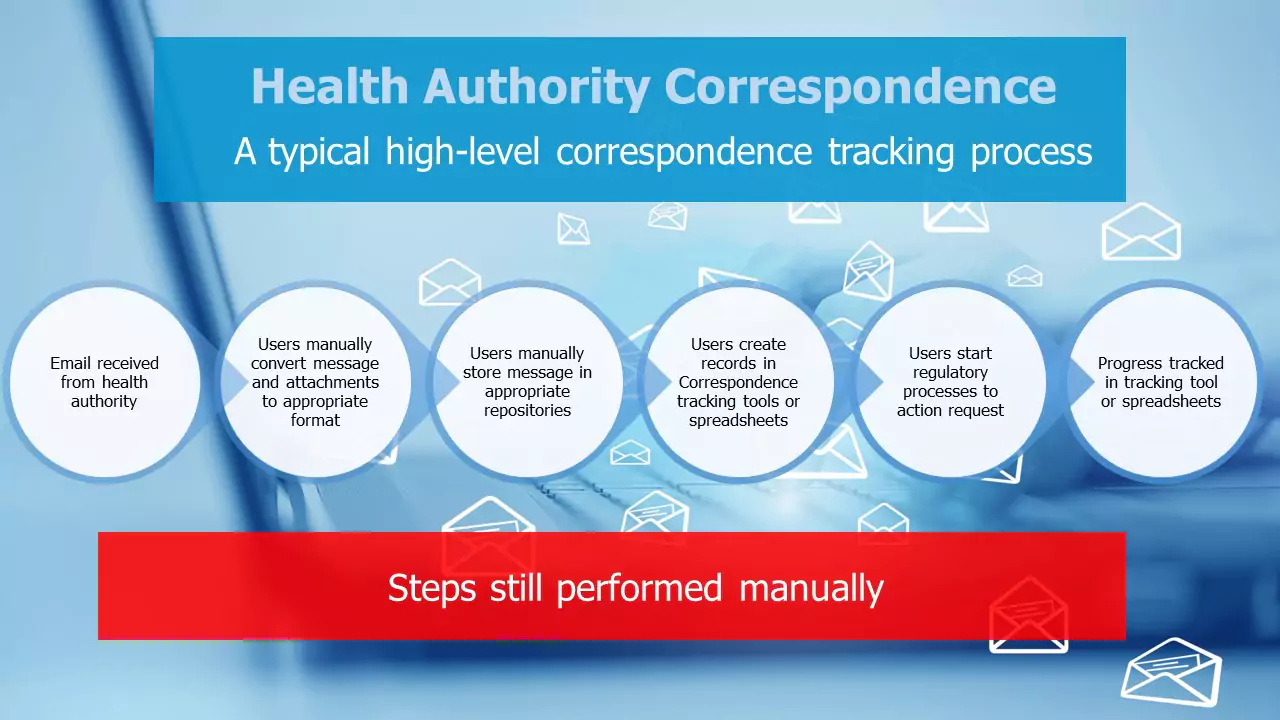
– Monitor incoming emails to identify incoming correspondence that relates to ongoing or future regulatory activities. Examples of this might be letters of approval, requests for more information, etc.
– Convert the identified emails and any attachments (supporting information in many different formats) identified into a useful, future-proof and standard format (such as PDF).
– Store the email and converted attachments in an appropriate location of a central repository (such as an electronic Document Management System).
– Create entries in the correspondence tracking system (if it exists, and if not in a tracking spreadsheet) to monitor the information received and any activities that are required to be completed due to the request.
How to reduce risks in your correspondence automation process?
The risks associated with manually tracking correspondence can be reduced. By automation and simplification.
DocShifter can help streamline the process and reduce the risks associated with correspondence tracking by automatically:
1. Monitoring email systems (or specific folders or other content storage repositories) for correspondence that needs to be processed.
2. Transforming the correspondence into a consumable format (such as fully compliant industry standard PDF) along with any attachments.
3. Storing the transformed content and/or original correspondence into the right repository.
4. Emailing individuals with the content or to alert them of the arrival of the correspondence.
5. Kicking off a task through the DocShifter API to start the entries in the correspondence tracking system.
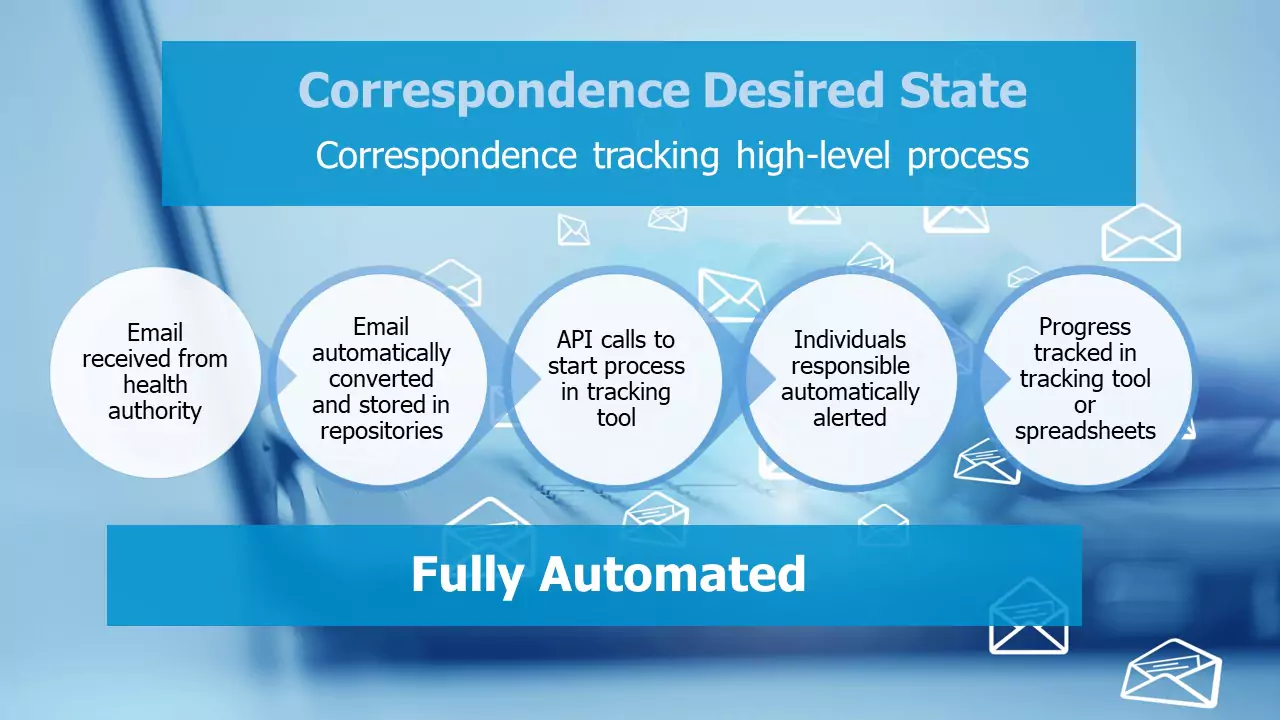
The historic pain points of correspondence tracking can be drastically reduced. In the meantime, read more about the correspondence tracking solution here.
Did you enjoy this blog post? We have more free knowledge-articles available for you.
Thank you very much for reading our blog post, we hope you enjoyed it.
For more free articles on regulatory topics (eCTD, regulatory submissions, RIM, eCTD v4.0, etc.) we invite you to join our free LinkedIn Regulatory Community.
This community is where we share tips & tricks, updates and learnings on regulatory topics . The community currently has 2000 members and is growing quickly.
In the first 2 editions, we focused on:
If you think this is interesting, I would like to personally invite you to join the group. And don’t worry, it is completely free.
About DocShifter – Correspondence tracking automation software
Speed, quality, scalability, and configurability are reasons why biotech, pharma and medical devices companies choose DocShifter to automate and simplify all their document conversion processes. From automatically checking and fixing Word and PDF documents to generating compliant, submission-ready PDF renditions, DocShifter software offers a unique and proven approach to speed up time-to-market.
High volume, high-quality document conversion, on-premise, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce Risk. Reduce IT infrastructure costs. Rated 5 stars on Gartner’s Capterra platform.
Bonus: We created an FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.



